Classification of Metals on the Basis of Energy Bands:
When the atoms come together to form a solid they are so close to each other that the fields of electrons of outer orbits from neighboring atoms overlap. This makes the nature of electron motion in a solid very different from that in an isolated atom. Inside the solid, each electron has a unique position, and no two electrons have same pattern of surrounding charges.
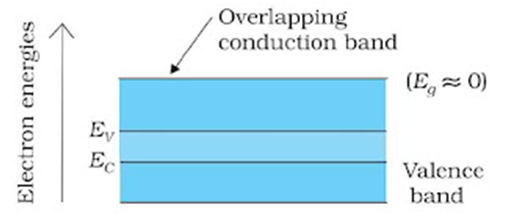
Metals: In metals, conduction band and valence band are overlapped to each other. The electrons from the valence band can easily move into the conduction band. Normally, the conduction band is empty but when it overlaps on the valence band, electrons can move freely into it, and it conducts electric current through it.
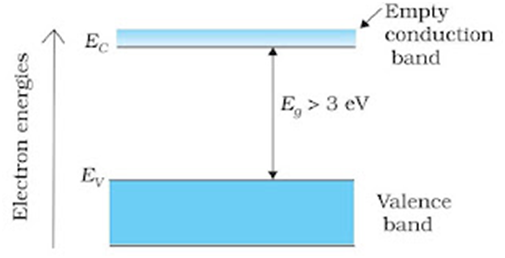
Semiconductors: Semiconductors are the core fundamental materials which are used in solid-state electronic devices such as transistors, diodes etc. The material’s atomic structure decides whether the material will turn out to be a metal, semiconductor, or insulator. Semiconductors could also be elements such as Ge, Si or compounds such as CdS or GaAs.
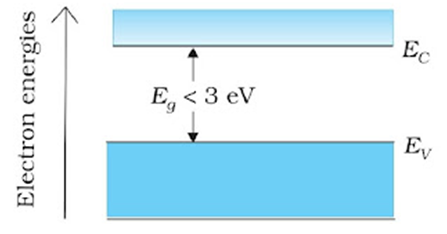
Insulators: In semiconductors, a small and finite energy band gap exists. Because of the small energy band gap some electrons from valence band, at room temperature, acquire enough energy to cross the energy gap and enter the conduction band. These electrons are very few and can move in the conduction band. Hence, the resistance of semiconductors is not as high as that of the insulators.
Intrinsic Semiconductor (Pure): The pure semiconductors in which the electrical conductivity is totally governed by the electrons excited from the valence band to the conduction band and in which no impurity atoms are added to increase their conductivity are called intrinsic semiconductors and their conductivity is called intrinsic conductivity. Electrical conduction in pure semiconductors occurs by means of electron-hole pairs. In an intrinsic semiconductor,
ne = nh = ni where ne = the free electron density in conduction band, nh = the hole density in valence band, and ni = the intrinsic carrier concentration
Extrinsic Semiconductors (Impure): A Semiconductor doped with suitable impurity atoms so as to increase its conductivity is called an extrinsic semiconductor.
Extrinsic semiconductors are basically of two types:
n-type semiconductors:
The pentavalent impurity atoms are called donors because they donate electrons to the host crystal and the semiconductor doped with donors is called n-type semiconductor. In n-type semiconductors, electrons are the majority charge carriers and holes are the minority charge carriers. Thus, ne≫nh
p-type semiconductors:
The trivalent impurity atoms are called acceptors because they create holes which can accept electrons from the nearby bonds. A semiconductor doped with acceptor type
impurities is called a p-type semiconductor. In p-type semiconductor, holes are the majority carriers and electrons are the minority charge carriers .
nh≫ne
