Introduction
In this chapter, we shall discuss some basic principles and techniques of analysis needed for understanding the formation and properties of organic compounds. Organic compounds are essential for existence and maintenance of life on earth. These include complex molecules like (DNA) which carry genetic information and proteins which is building blocks of life. Organic compounds also play an important role in material used in daily life such as cloths, fuel, dyes, and medicines etc.
Structural Representations of Organic Compounds
Structural Formulas
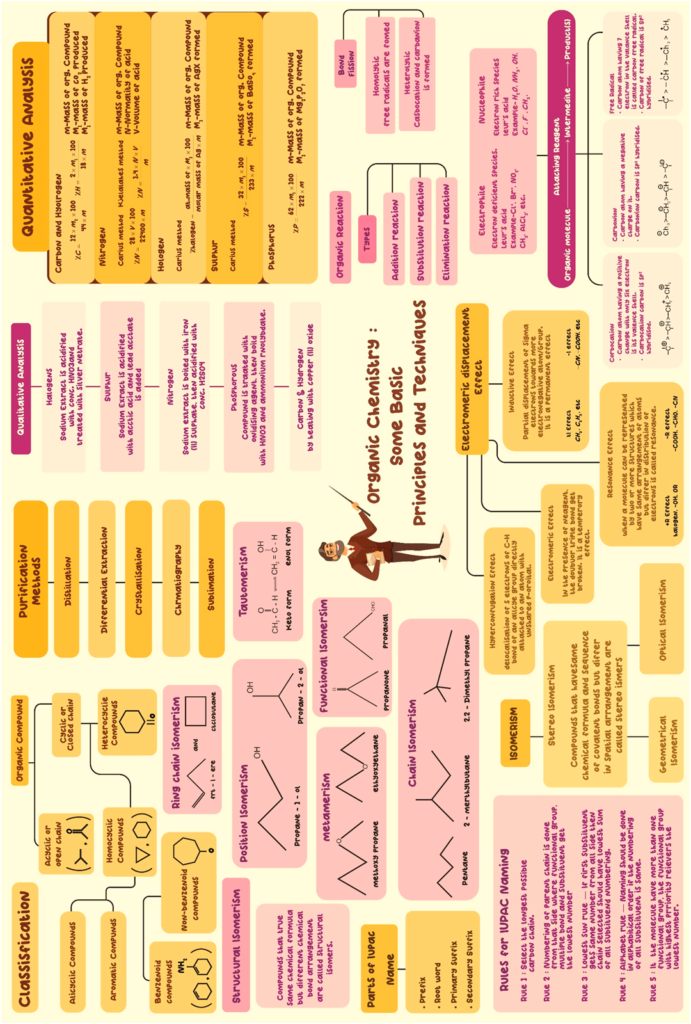
The Lewis structures can be simplified by representing the two electron covalent bonds by a dash (–). In this representation, a single bond is represented by a single dash (–), a double bond by a double dash (=) and a triple bond by a triple dash (≡). The lone pair on an atom may or may not be shown. This representation is called structural formula.
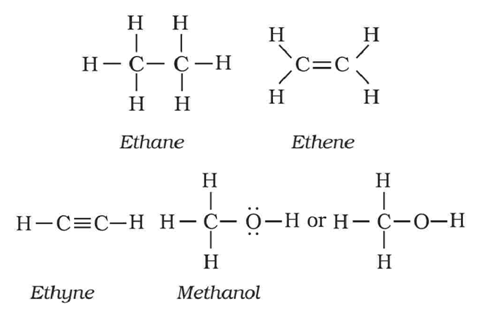
Condensed Formulas
In this formula, the arrangement of atoms are shown but the bonds between may be omitted and the number of identical groups attached to an atom are indicated by a subscript.
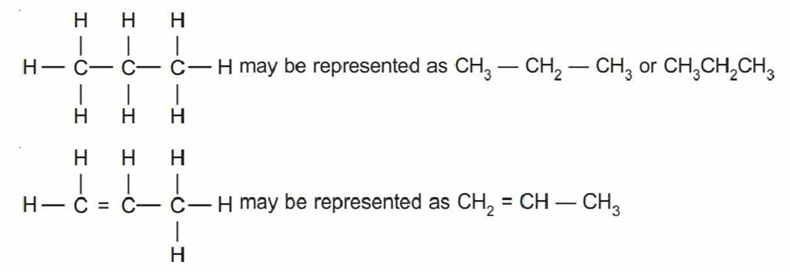
Condensed Formulas
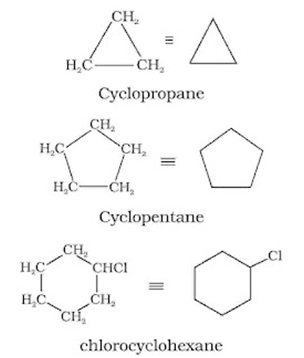
In this representation, the carbon and hydrogen atoms are not shown and the lines between carbon-carbon bonds are shown in a zig-zag manner.
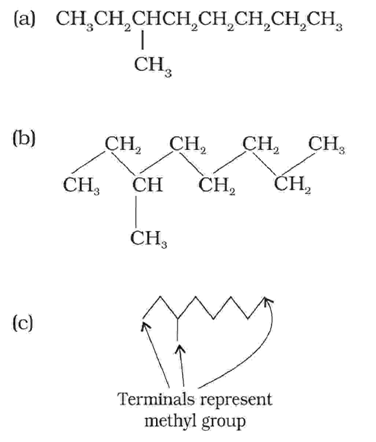
In cyclic compounds, the bond-line formulas may be given as follows:
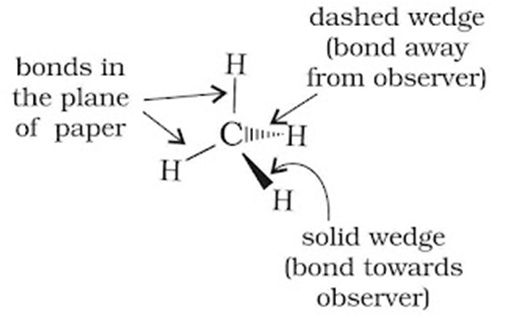
Three-dimensional representation of organic molecules
The three-dimensional (3-D) structure of organic molecules can be represented on paper by using certain conventions. In these formulae, the thick solid (or heavy) line or the solid wedge indicates a bond lying above the plane of the paper and projecting towards the observer while a dashed wedge is used to represent a bond lying below the plane of the paper and projecting away from the observer.
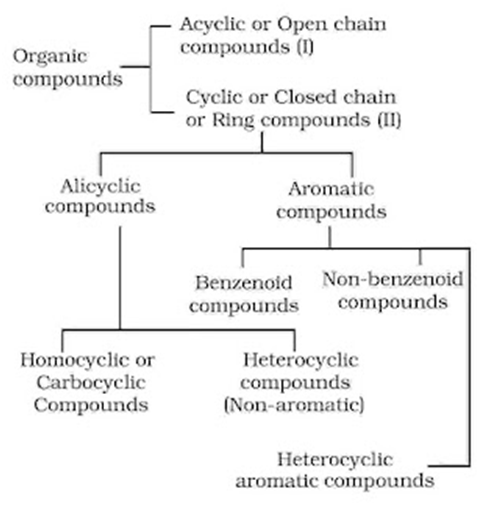
Classification of Organic Compounds
On the basis of their structures, organic compounds are broadly classified as follows:
Open Chain Compounds
These compounds contain open chains of carbon atoms in their molecules. The carbon chains may be either straight chains or branched chains. They are also called aliphatic compounds.
Closed Chain or Ring Compounds
These compounds contain chains or rings of atoms in their molecules.
Alicyclic Compounds: These compounds contain a ring of three or more carbon atoms in them. They resemble aliphatic compounds in many of their properties.
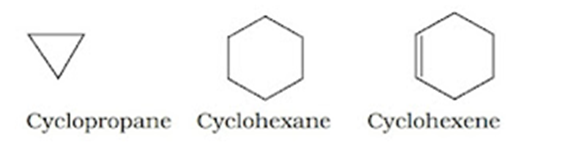
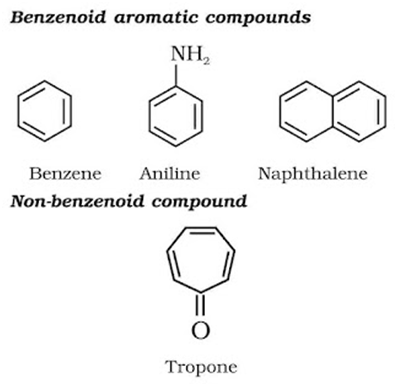
Aromatic Compounds: These have a cyclic system containing at last one benzene ring. The parent member of the family is called benzene. Benzene has a homocyclic hexagonal ring of six carbon atoms with three double bonds in the alternate positions.
Aromatic Compounds: These have a cyclic system containing at last one benzene ring. The parent member of the family is called benzene. Benzene has a homocyclic hexagonal ring of six carbon atoms with three double bonds in the alternate positions.
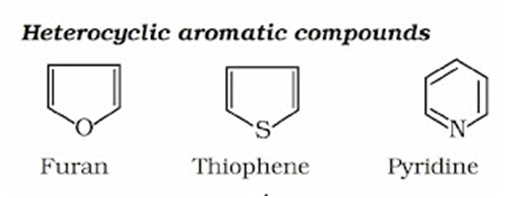
Heterocyclic Compounds: In these compounds, the ring contains one or more atoms of either nitrogen, oxygen or sulphur in addition to carbon atoms. The atom other than carbon (such as N, O, S) present in the ring is called hetero atoms.
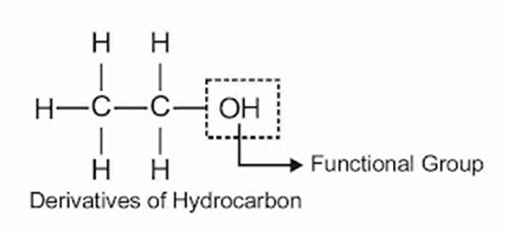
Functional Groups: An atom or group of atoms which largely determines the properties of the organic compounds particularly the chemical propertiesFunctional Groups: An atom or group of atoms which largely determines the properties of the organic compounds particularly the chemical properties
Homologous Series: Homologous series may be defined as “a series of similarly constituted compounds in which the members possess the same functional group and have similar chemical characteristics”. The two consecutive members differ in their molecular formula by –CH2– group.
- CH3OH – Methyl alcohol
- C2H5OH – Ethyl alcohol
- C3H7OH – Propyl alcohol
- C4H9OH – Butyl alcohol
- C5H11OH – Pentyl alcohol
- C6H13OH – Hexyl alcohol
Nomenclature of Organic Compounds
The term ‘nomenclature’ means the system of naming of organic compounds. There are two systems of nomenclature:
- Trivial or Common System
In this nomenclature, the names of organic compounds were assigned based on their source of origin or certain properties. For instance, citric acid got its name from the source (citrus fruits) from which it was first isolated. Formic acid was named so as it was first obtained from red ant. In Latin ant word is formica.
- IUPAC System of Nomenclature
A systematic method of naming has been developed and is known as the IUPAC (International Union of Pure and Applied Chemistry) system of nomenclature. In this systematic nomenclature, the names are correlated with the structure such that the reader or listener can deduce the structure from the name.
Summary–
- Condensed Structural Formula: The structural formulae obtained by omitting some or all the covalent bonds and by indicating the number of identical groups attached to an atom by subscript is called condensed structural formula.
- Bond-line Structural Formula: In this formula of organic compounds, carbon and hydrogen atoms are not shown and line representing C –C bonds and drawn in zig-zag fashion. The only atoms specifically written are those that are neither nor hydrogen bonded to carbon.
- Cyclic Compounds: These are compounds in which carbon atoms are joined in rings i.e., they are closed chain compounds.
- Aromatic Compounds: Benzene and its derivatives are called aromatic compounds.
- Functional group: Functional group is an atom or group of atoms or reactive part of the compound which determines physical and chemical properties of compounds.
- Homologous Series: Homologous series is a series of compounds which has same functional group same general formula and show gradation in physical and chemical properties of compounds.
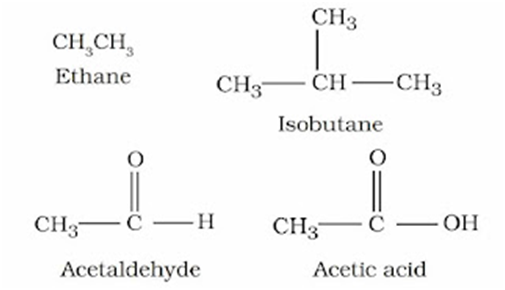
- Isomerism: The phenomenon of existence of two or more compounds possessing the same formula but different structural formula and different physical and chemical properties are called isomerism.
- Structural Isomerism: Compounds having the same molecular formula but different structures are classified as structural isomers. Chain Isomerism: The isomers, which differ in carbon atom chain, are called chain isomers and this phenomenon is called chain isomerisms.
- Position Isomerism: The isomers, which differ in position of substituent or functional groups are called position isomers and this phenomenon is called position isomerism.
- Functional Isomerism: Those isomers, which differ in functional groups are called functional isomers and this phenomenon is called functional isomerism.
- Metamerism: Those isomers, which differ in alkyl group attached with the di or tri valent atom of functional group. These are called metamers and this phenomenon is called metamerism.
- Stereoisomerism: Those compounds that have the same composition and sequence of covalent bond but differ in relative positions of their atoms or groups in space.
- Free Radical: An atom or group of atoms containing odd unpaired electrons in excited state is known as free radical.
