Most of the substance around us undergoes various changes. Some of these changes are temporary with no new substance being formed. They are called physical changes.
Example: Water changes to steam on boiling but no new substance is formed(Even though steam and water look different when they are made to react with a piece of Na, they react the same way and give the exact same products). This involves only a change in state (liquid to vapour).
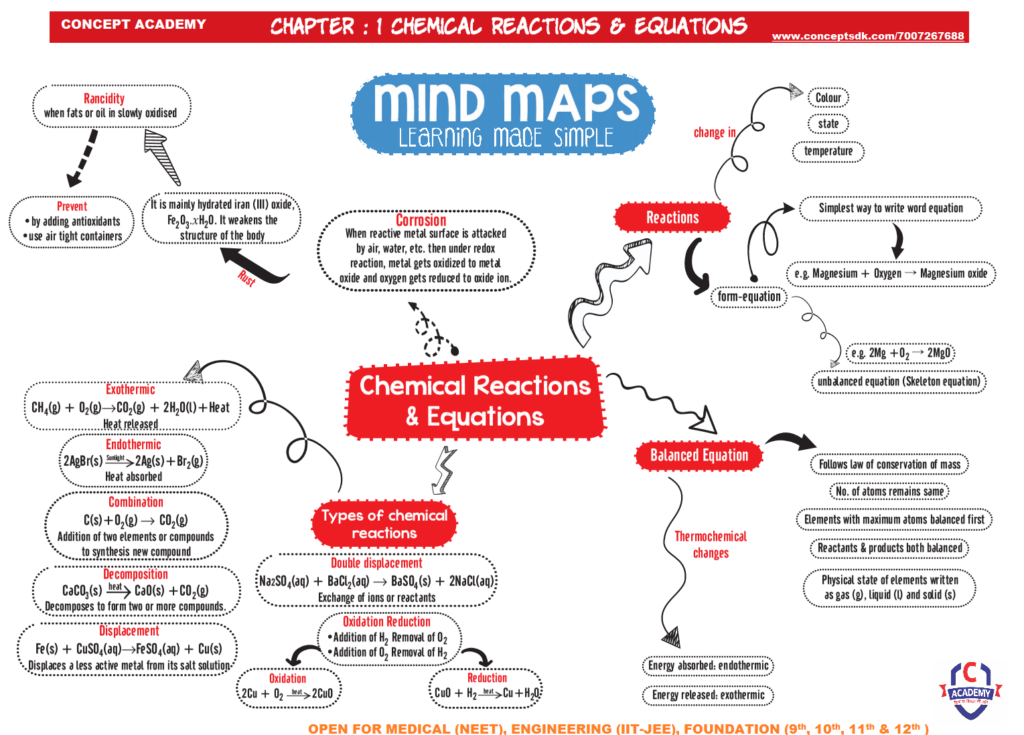
A substance is said to undergo a chemical change when the chemical properties of a substance alter. As a result, there is either formation or breaking of atomic bonds at the molecular level. Some characteristics of a chemical change are:
New substances are produced during a chemical reaction.
Changes in energy are involved.
During the reaction, there occurs a change in mass.
There is a permanent alteration.
